27. DMG +NiCl2+NH4OH makes Complex a+ NH4Cl+H2O. What is complex a and find the hybridisation magnetic character and Oxidation state of Nickel in complex a ?
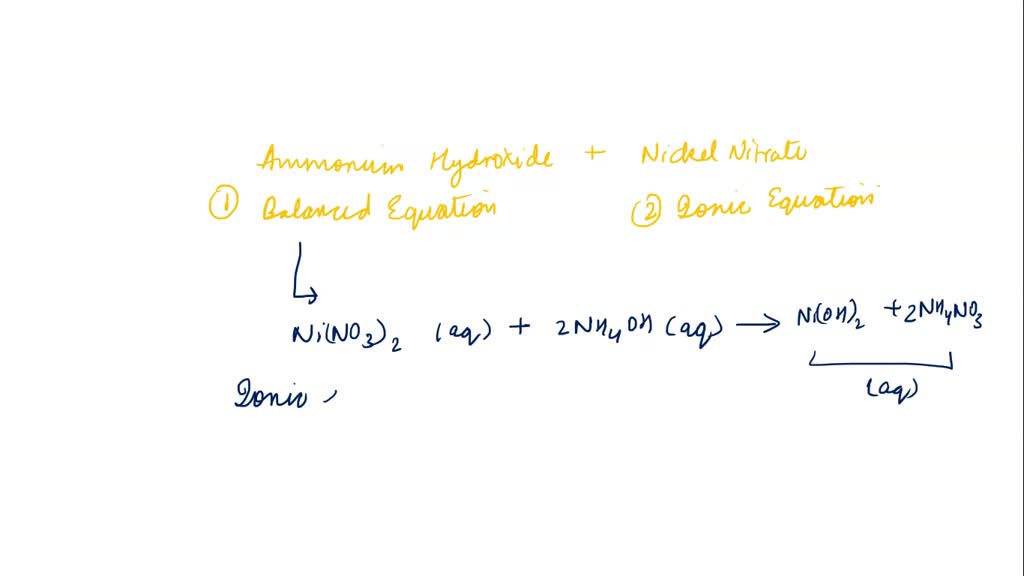
SOLVED: When an aqueous solution of NH4OH is mixed with an aqueous solution of Ni(NO3)2, a pale yellow precipitate forms. Write a balanced molecular equation for this reaction. Write the complete ionic
29. NiCl2 + NH4OH + dimethylglyoxime ——> A ( complex ) Incorrect statement for complex A is /are 1 Coordination number of metal ion is 4 2 Two five membered and two

Scanning Electron Microscope image of Ni-BTC MOFs. (a) Ni-BTC Anl ; (b)... | Download Scientific Diagram

Alkaline leaching of nickel bearing ammonium jarosite precipitate using KOH, NaOH and NH4OH in the presence of EDTA and Na2S | Semantic Scholar

It is an experimental fact that:- DMG+Ni(II) salt+NH_4OH→ Red precipitate Which of the following ... - YouTube

Effect of temperature on Ni and Cd leaching recovery (NH4OH / (NH4)2CO3... | Download Scientific Diagram

E740: Equilibrium – Complex Ions – Metal + Ammonia Complexes | Lecture Demonstration Manual General Chemistry | University of Colorado Boulder

E735: Complex Ions and Precipitates – Nickel(II) compounds | Lecture Demonstration Manual General Chemistry | University of Colorado Boulder

Shape-controlled synthesis of Ni(OH)2/NiO nanowalls by surface reaction of Ni foil in aqueous NH4OH - ScienceDirect
![A 1L solution contains 0.2 M NH4OH and 0.2 M NH4Cl . If 1.0 mL of 0.001 M HCl is added to it, what will be the [OH^-] of the resulting solution? ( Kb = 2 × 10^-5 ) A 1L solution contains 0.2 M NH4OH and 0.2 M NH4Cl . If 1.0 mL of 0.001 M HCl is added to it, what will be the [OH^-] of the resulting solution? ( Kb = 2 × 10^-5 )](https://dwes9vv9u0550.cloudfront.net/images/4552108/6bc4141a-e782-46b7-a1b8-65fa8d179f7a.jpg)
A 1L solution contains 0.2 M NH4OH and 0.2 M NH4Cl . If 1.0 mL of 0.001 M HCl is added to it, what will be the [OH^-] of the resulting solution? ( Kb = 2 × 10^-5 )

SOLVED: A nickel coordination compound, Ni(NH3)6Cl2, can be prepared by a procedure similar to the one you will use to prepare Cu(NH3)4SO4·H2O. The reaction can be represented stoichiometrically as NiCl2·6H2O + 6





/NH4OH%20Fusion%20Chemical%20Blending%20System.jpg?width=1305&name=NH4OH%20Fusion%20Chemical%20Blending%20System.jpg)



